Section outline
-
H. Tuğba Doğmuş Lehtijarvi1, Anna Maria Vettraino2, Nikoleta Soulioti3, Asko Lehtijarvi1, Steve Woodward1
1Isparta University of Applied Sciences (ISUBU), Faculty of Forestry, Isparta, Turkey
2Department for Innovation in Biological Systems, Agriculture and Food (DIBAF), University of Tuscia, Viterbo, Italy
3Institute of Mediterranean Forest Ecosystems, Terma Alkmanos, 11528 Athens, Greece, Institute of Mediterranean Forest Ecosystems, Athens, Greece




-
-
Preferred name: Ceratocystis platani
Authority: (J.M. Walter) Engelbrecht & Harrington
Taxonomic position: Fungi: Dikarya, Ascomycota: Pezizomycotina: Sordariomycetes: Hypocreomycetidae: Microascales: Ceratocystidaceae
Other scientific names: Ceratocystis fimbriata f. sp. platani Walter, Endoconidiophora fimbriata f. platani Walter
Common names in English: canker of sycamore (US), canker stain of plane
-
-
-
In urban settings, Platanus are often planted as a single species along avenues or in park settings. Following infection with Ceratocystis platani, the first symptoms to appear are chlorotic and wilting foliage on a single branch, possibly along with sap exudation from cracks in the branch and stem bark. Such symptoms are more likely to be seen in avenue plantings (Figure 1. a, b), as in parkland or woodland settings (Figure 1. c, d), vision can be obscured by surrounding trees. Close inspection will reveal stem/branch cankers (lesions) Figure (2. a, b). Over time, the necrotic bark turns pale-brown and cracks, but remains attached to the tree; no wound callus forms at the lesion margins. When cutting away the bark beyond the lesion, orange/brown staining is visible in the inner bark and vascular cambium. These symptoms are most marked at the upper and lower edges of the lesion. As lesions girdle the trunk or a main branch, the bark beyond the lesions turns reddish-brown as the tissues die. In cross-section, affected branches show bluish-black, then brown, spindle-shaped patches, extending radially and more or less side by side (Figure 2. c). In the highly susceptible host trees found in Europe and Eurasia, infections are always lethal, although trees can take from a few months to 2.5 – 3 years to die, depending on size and pre-infection vigour. Further details are available elsewhere (Walter, 1946; Griffin, 1968; Vigouroux, 1979a; Panconesi, 1981; Tsopelas et al., 2017).
Earlier in infection development, sap exudation can be observed from small cankers, visible as dark staining in tissues below cracks in the bark surface. The exudation becomes oxidized, turning dark brown. Small cankers are more obvious on smooth-barked species, such as P. occidentalis and P. x hispanica than on the rough bark of P. orientalis (Panconesi, 1981; Walter et al., 1952).

-
-
-
Entry to host trees occurs through wounds into bark and sapwood. Infected superficial wounds lead to rapid colonization of the inner bark (secondary phloem), vascular cambium and sapwood, with longitudinal spread of up to 2 – 2.5 m per year (in P. x hispanica) in the sapwood xylem; Further growth of C. platani at a rate of 50 – 100 cm per year occurs in the inner bark, causing dark staining in those tissues and the underlying sapwood. The fungus also grows through medullary rays, sometimes reaching the heartwood.
Growth of C. platani can occur between 10 and 45°C, but the fungus survives at temperatures as low as -17°C. Optimum temperature for growth is 25°C. In the soil, asexual spores may survive for at least 105 days during winter, but are killed when soil temperatures exceed 35-40°C (Accordi, 1989).
Another common method of transmission from infected trees to adjacent healthy trees is via root-to-root contact, which is common in many Platanus species, especially in the (largely) clonal P. x acerifolia (Accordi, 1986). Insect transmission is common in the Ceratocystidaceae (de Beer et al., 2014) and other ophiostomatoid fungi (Wingfield et al., 1993), and there is some evidence that bark boring and other insects may carry C. platani between trees, at least in in riparian ecosystems (Crone, 1962; Soulioti et al., 2015); this possibility requires further verification. In urban trees, C. platani is frequently transmitted via pruning wounds and damage caused by other human activities on and around the trees (Tsopelas et al., 2017). Sawdust from diseased trees is highly infective, and is probably the main method of transmission in urban Platanus, particularly those that are pruned regularly.
None of the spore types produced by C. platani appears to be airborne under natural conditions (Panconesi, 1999), but the pathogen certainly spreads locally on infected sawdust generated during pruning and sanitation felling operations. If these operations occur near a watercourse, infected sawdust may be carried downstream and lead to infections of riparian Platanus trees. Human activities are, therefore, the main mechanisms of spread.
Natural spread from tree to tree via root contacts, although slow, is of importance in infection foci. Terracing machinery used in construction activities has been demonstrated to carry infested soil and contaminate previously healthy areas. Spread of the fungus may also occur in colonized Platanus timber, in countries where this is used: this pathway is probably how the pathogen was spread from North America to Italy (Panconesi, 1999; Tsopelas et al., 2017). Trade in symptom-free Platanus may inadvertently transfer infected host plants internationally. The most significant pathways for transfer of C. platani into new areas are likely to be host plants for planting, wood (e.g., timber, wood packaging material, wood chips, dunnage, firewood) and machinery (EFSA, 2016).
-
-
-
Cultures of C. platani are at first hyaline and more or less dense, depending on the culture medium, becoming brownish-green and giving off a pronounced banana smell, the intensity of which varies with culture medium. Growth is rapid (5 mm in 24 h at 24°C on potato dextrose agar). Perithecia (diam: 200 µm) have long necks (400-800 µm), although not all isolates produce these structures, while in others, aborted perithecia, never attaining maturity, are formed. Ascospores (diam: 4-8 µm) have distinctive bowler hat shapes.
In culture, three types of conidia form: (1) hyaline truncated cylindrical endoconidia (5-40 x 3-6 µm) in long rigid arched chains on 60-90 µm long conidiophores, produced on an approximately daily cycle; (2) more rarely, doliform endoconidia form, very pale in colour, dimensions of 7-12 x 6-9 µm, in short chains; (3) thick-walled endoconidia (chlamydospores) are bulbous, brownish-green, 11-19 x 9-15 µm. Conidia are very numerous in infected wood (and thus in sawdust). For more information, see Hunt (1956), Webster & Butler (1967), Ferrari & Pichenot (1974).
-
-
-
Ceratocystis platani can be baited from infected wood or frass within 48 h by placing colonized tissues in moist chambers or on pieces of autoclaved carrot in Petri dishes at 20 - 25°C and incubation for 24-48 hours, when asexual spores are visible; after approximately 7 days, ascomata form (Vigouroux, 1979b; Ocasio-Morales et al., 2007). In culture, the fungus grows well on both malt extract agar or potato dextrose agar. A trap/bait technique (Grosclaude et al., 1988) is effective in isolating the pathogen from soil or infected wood: the bark is removed from healthy twigs or branches of Platanus orientalis or P. x hispanica before placing in close contact with the wood or soil sample and incubating in a humid chamber or in water at room temperature. Perithecia become visible on the exposed sapwood of branches within a few days. A similar technique can detect C. platani in river water.
Molecular methods are available for the specific detection and identification of C. platani without a requirement for isolation. A quantitative PCR technique (Pilotti et al., 2012; Luchi et al., 2013) detected C. platani DNA in low quantities and with high precision from sample traps placed in the vicinity of infected trees in Italy. Ceratocystis platani DNA was detected as far as 200 m from trees felled for sanitation purposes in Firenze, using real-time PCR (Luchi et al., 2013). These methods are useful in monitoring the spread of the disease in a region.
An EPPO Diagnostic Protocol for C. platani provides recommendations for the detection and identification of the pathogen in plant material, soil or water samples (EPPO Standard PM 7/14, 2014).

-
-
-
The only known hosts of C. platani are species in the genus Platanus: P. x hispanica (=P. x acerifolia), widely planted as an amenity tree in many parts of western Europe, and the European/ Eurasian parent P. orientalis, are the species most severely affected (Panconesi, 1981). The North American parent of P.x hispanica, P. occidentalis is susceptible to some dieback but is not usually killed by infection (McCracken & Burkhard, 1977). Platanus racemosa dieback and mortality were recorded in street trees of Modesto, California (Perry and McCain, 1988), but infections have not been found on other North American species of Platanus, or on P. kerrii which occurs in Laos and Vietnam.
Platanus occidentalis, Platanus orientalis, Platanus racemosa, Platanus x hispanica
-
-
-
Quarantine
Spread mainly results through activities of humans, and strict, standard horticultural propagation and production methods will limit dispersal (Smith, 1985). Planting material should be obtained from tree nurseries situated in disease-free regions: plants must be raised in locations known to be free from C. platani during (at least) the last growing season. Tools should be disinfected with alcohol or another high-potency disinfectant before pruning operations, even in uninfected regions, and should be repeated between each tree, particularly in infected regions. Any diggers or other hydraulic machinery used in the proximity of infected Platanus should be treated with an approved fungicide in pressurized water before being moved off that site (Blankart & Vigouroux, 1982), as adhering infective propagules may be transferred over considerable distances on machinery (Tsopelas & Soulioti, 2014). When infected trees are felled, all debris and sawdust should be sprayed to run-off with fungicide before sweeping up and disposal. Any potentially infected wood and other debris should be burned; transporting infected firewood to disease-free areas should be actively discouraged.
Apart from phytosanitary measures, no simple control methods are available. Breeding for resistance and related research produced a hybrid of P. x hispanica showing greater resistance, named ‘Vallis Clausa’(Vigouroux & Olivier, 2004), but more work is required to find the wide range of resistant host genotypes required for wide deployment in invaded environments, particularly given the extreme susceptibility of P. orientalis.
European Regulatıon (EU) 2022/1629 of 21 September 2022, implementing previous regulations, including European Regulation 2016/2031. Regulation (EU) 2020/1231, established that annual surveys for the presence of the disease should be carried out to ensure early detection of the pathogen in areas of the Union territory where the specified pest is not known to be present. Further details can be found at:https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R1629
-
-
-
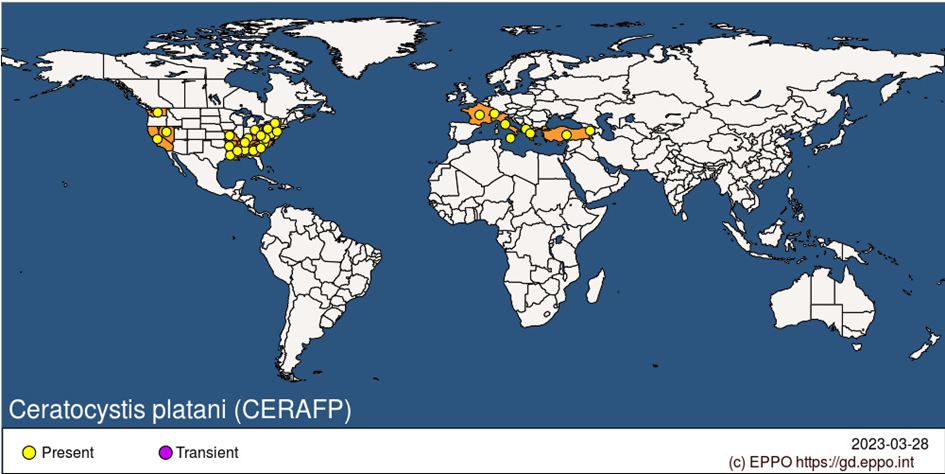
Ceratocystis platani was most likely introduced into several southern European ports from the USA at the end of the Second World War on infected wood packaging material, after which it spread rapidly in Italy (Panconesi, 1981) and more slowly in France (Vigouroux, 1979a). Initially, the western part of Vaucluse (south-eastern France) was severely affected. Recently, infected 200-years old P. x hispanica along the Canal du Midi in south-western France were felled, which received considerable media attention. The disease was reported several times in Spain, but only confirmed in 2010 in Girona, Catalonia, in a region bordering affected areas of France (EPPO, 2014; Riba, 2011), an outbreak now considered eradicated. The disease is known in Switzerland, Greece, Albania and Turkey. Unverified reports suggest that the disease is also present in Armenia (Simonian and Mamikonyan, 1982) and Iran (Salari et al., 2006). Abundant dying and dead P. orientalis were observed in valleys approaching the North Macedonian border in 2022 (Doğmuş & Woodward, 2022, personal observations), suggesting that spread is continuing in the Balkans Peninsula.
EPPO Region: Albania, Armenia, France (mainland), Greece (mainland), Italy (mainland, Sicilia), Switzerland, Türkiye.
North America: United States of America (Alabama, Arkansas, California, Delaware, District of Columbia, Georgia, Kentucky, Louisiana, Maryland, Mississippi, Missouri, Nevada, New Jersey, New York, North Carolina, Ohio, Pennsylvania, South Carolina, Tennessee, Virginia, Washington, West Virginia).
-
-
-
During the first known outbreaks in the eastern USA, large numbers of planted Platanus trees, mostly P. x hispanica, were lost in cities over a 20 – 30-year period (Walter et al., 1952; Crone, 1962). In south-eastern France, around Marseille, thousands of street trees were killed by C. platani (Ferrari & Pichenot, 1974; 1976). The disease soon spread into the neighbouring department of Vaucluse, where an estimated 1,500 to 1,700 infected and adjacent trees were felled annually; more than 30,000 trees were removed in Provence Alpes Côte d’Azur over 25 years (Chapin and Arcangioli, 2007). More recently, the problem with the disease along a substantial part of the Canal du Midi, which connects the Atlantic Ocean to the Mediterranean Sea through western France, received media attention, not least because of the high costs of removal and replacement. Initial findings of C. platani were near Toulouse in the early 2000s (Bonnet and Collet, 2007), after which the pathogen spread quickly, possibly in the Canal du Midi itself. Along the Canal du Midi, some 10,000 to 13,000 trees were diagnosed as infected and felled (VNF, 2019).
The fungus spread from ports into the north of Italy in short time after introduction, killing many trees, particularly those newly planted along avenues (Panconesi, 1981; 1999). There was a big outbreak in and around Naples after the end of World War II, coinciding with American Service bases, with losses amounting to 90 % of street trees by the end of the 1980s (Panconesi, 1999). By that time, the disease was present throughout the Italian peninsula, affecting trees in all major cities (Panconesi, 1999).
The disease was discovered in natural stands of P. orientalis in Sicily in the late 1980s (Granata & Pennisi, 1989), and was soon followed by reports from Greece, probably arriving there in the late 1990s (Tsopelas & Angelopoulos, 2004). Platanus orientalis is a common, native tree in Greece, particularly in riparian zones. The pathogen established in the Peloponnesos, but rapidly spread into north-western Greece and, subsequently, beyond that region. The high level of susceptibility of P. orientalis to C. platani led to devastation of natural populations of the tree, and the spread into Albania (Tsopela et al. 2015), with significant mortality in natural ecosystems and cities, shows that it threatens other Balkan countries and countries to the east.
In Türkiye, further spread occurred into the European part of Istanbul, where Platanus are important planted trees in urban parks and streets (Lehtijärvi et al., 2018), presumably due to the import of infected trees.
-
-
-
1. Accordi SM (1986) [Spread of Ceratocystis fimbriata f.sp. platani through root anastomoses]. Informatore Fitopatologico 36, 53-58. [In Italian].
2. Accordi SM (1989) [The survival of C. fimbriata f.sp. platani in the soil]. Informatore Fitopatologico 39, 57-62. [In Italian].
3. Blankart D, Vigouroux A (1982) Lutte contre le chancre coloré du platane. Phytoma No. 343, p. 51.
4. Bonnet R, Collet E (2007) Gestion preventive du chancre coloré sur des plantations de platanes en situation humide - exemple du canal du Midi, pp 72-82. In: Colloque national, Chancre coloré du platane. 11 Octobre 2007. ENSAT, Toulouse, France.
5. Chapin E, Arcangioli D (2007) Évolution et situation du chancre coloré dans le monde, en Europe et en France, pp 9-20. In: Colloque national. Chancre coloré du platane. 11 Octobre 2007. ENSAT, Toulouse, France.
6. Regulation (EU) 2022/1629 of 21 September 2022 establishing measures for the containment of Ceratocystis platani (J.M. Walter) Engelbr. & T.C. Harr. within certain demarcated area. Official Journal of the European Union L 245/14
7. Crone LJ (1962) Symptoms, spread, and control of canker stain of plane trees. Ph.D. Thesis, Rutgers University, New Brunswick, NJ.
8. de Beer ZW, Duong TA, Barnes I, Wingfield BD, Wingfield MJ (2014) Redefining Ceratocystis and allied genera. Studies in Mycology 79, 187-219.
9. EPPO (2014) EPPO Standard. Diagnostics. PM 7/14(2) Ceratocystis platani. EPPO Bulletin 44, 338–349.
10. EFSA (2016) EFSA PLH Panel. Jeger M, Bragard C, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Santini A, Tsopelas P, Vloutoglou I, Pautasso M, Rossi V. Scientific opinion on the risk assessment and reduction options for Ceratocystis platani in the EU. EFSA Journal 14(12), 4640, 65 pp. https://doi.org/10.2903/j.efsa.2016.4640
11. Ferrari JP, Pichenot M (1974) Ceratocystis fimbriata responsable d'une grave maladie du platane en France: la tache chancreuse. Compte Rendu des Séances de l'Académie des Sciences 278, 2787-2789.
12. Ferrari JP, Pichenot M (1976) The canker stain disease of plane tree in Marseille and in the south of France. European Journal of Forest Pathology 6, 18-25.
13. Granata G, Pennisi AM (1989) Estese morie di platani orientali in forestazioni naturali causate da Ceratocystis fimbriata (Ell. et Halst.) Davidson f. platani Walter. Informatore Fitopatologico 12, 59-61.
14. Griffin HD (1968) The genus Ceratocystis in Ontario. Canadian Journal of Botany 46, 689-718.
15. Grosclaude C, Olivier R, Pizzuto JC, Romiti C, Madec S (1988) Detection of C. fimbriata f.sp. platani by trapping. Application to the study of the persistence of the parasite in infected wood. European Journal of Forest Pathology 18, 385-390.
16. Hunt J (1956) Taxonomy of the genus Ceratocystis. Lloydia 19, 1-58.
17. Lehtijärvi A, Oskay F, Doğmuş HT, Aday Kaya AG, Santini A, Woodward S (2018) Ceratocystis platani killing Platanus trees in Istanbul, Turkey. Forest Pathology 47, e12375. https://doi.org/10.1111/efp.12375
18. Luchi N, Ghelardini L, Belbahri L, Quartier M, Santini A (2013) Rapid detection of Ceratocystis platani inoculum by quantitative real-time PCR assay. Applied and Environmental Microbiology 79, 5394–5404.
19. McCracken FI, Burkhard EC (1977) Destruction of sycamores by canker stain in the midsouth. Plant Disease Reporter 61, 984-986.
20. Ocasio-Morales R, Tsopelas P, Harrington TC (2007) Origin of Ceratocystis platani on native Platanus orientalis in Greece and its impact on natural forests. Plant Disease 91, 901-904.
21. Panconesi A (1981) Ceratocystis fimbriata of plane trees in Italy; biological aspects and control possibility. European Journal of Forest Pathology 11, 383-395.
22. Panconesi A (1999) Canker stain of plane trees: a serious danger to urban plantings. Journal of Plant Pathology 81, 3-15.
23. Perry E, McCain AH (1988) Incidence and management of canker stain in London plane trees in Modesto, California. Journal of Arboriculture 14, 18-19.
24. Pilotti M, Lumia V, Di Lernia G, Brunetti A (2012) Development of real-time PCR for in wood-detection of Ceratocystis platani, the agent of canker stain of Platanus spp. European Journal of Plant Pathology 134, 61–79.
25. Riba JM (2011) El chancro colorado del plátano: Ceratocystis fimbriata f. sp. platani; afectaciones en Girona. Boletín de la Asociación Española de Parques y Jardines 61, 6-10.
26. Salari AN, Arefipoor MR, Jami F, Zahedi M, Mehrabi A, Zeinali S (2006) First report of Ceratocystis fimbriata f. sp. platani causal agent of canker stain of sycamore trees in Iran, p 401. In: Proceedings of the 17th Iranian Plant Protection Congress, 2-5 September 2006. University of Tehran Karaj, Iran.
27. Simonian SA, Mamikonyan TO (1982) Disease of plane tree. Zashchita Rastenii 8, 23-24.
28. Smith IM (1985) Pest and disease problems in European forests. FAO Plant Protection Bulletin 33, 159-164.
29. Soulioti N, Tsopelas P, Woodward S (2015) Platypus cylindrus, a vector of Ceratocystis platani in Platanus orientalis stands in Greece. Forest Pathology 45, 367-372.
30. Tsopelas P, Angelopoulos A (2004) First report of canker stain disease of plane trees, caused by Ceratocystis fimbriata f. sp. platani in Greece. Plant Pathology 53(4), p 531.
31. Tsopelas P, Palavouzis S, Tzima AK, Tsopelas MA, Soulioti N, Paplomatas EJ (2015) First report of Ceratocystis platani in Albania. Forest Pathology 45, 433-436.
32. Tsopelas P, Santini A, Wingfield MJ, de Beer ZW (2017) Canker stain: A lethal disease killing iconic plane trees. Plant Disease 101, 645-658.
33. Tsopelas P, Soulioti N (2014) Invasion of the fungus Ceratocystis platani in Epirus: a potential threat of an environmental disaster in the natural ecosystems of plane trees. Phytopathologia Mediterranea 53, p 340.
34. Vigouroux A (1979a) Les 'dépérissements' des platanes: causes, importance, mesures envisageables. Revue Forestière Française 31, 28-39.
35. Vigouroux A (1979b) Une méthode simple de recherche de Ceratocystis fimbriata platani sur arbre en place. European Journal of Forest Pathology 9, 316-320.
36. Vigouroux A, Olivier R (2004) First hybrid plane trees to show resistance against canker stain (Ceratocystis fimbriata f. sp. platani). Forest Pathology 34, 307-319.
37. VNF (2019) Voies navigables de France Ministère de l’Environnement, de l’Énergie et de la Mer. Online report, retrieved 12 August 2020 from http://www.sudouest.vnf.fr/2014-vnf-replante-le-canal-et-le-chancre-colore-a432.html
38. Walter JM (1946) Canker stain of plane trees. US Department of Agriculture Circular No. 742.
39. Walter JM, Rex EG, Schreiber R (1952) The rate of progress and destructiveness of canker stain of plane-trees. Phytopathology 42, 236-239.
40. Webster RK, Butler EE (1967) A morphological and biological concept of the species Ceratocystis fimbriata. Canadian Journal of Botany 45, 1457-1468.
41. Wingfield MJ, Seifert KA, Webber JF (1993) Ceratocystis and Ophiostoma: Taxonomy, Ecology & Pathogenicity. St. Paul, Minnesota, The American Phytopathology Society, APS Press
-
-