Section outline
-
Tamara Corcobado
Austrian Research Centre for Forests (BFW), Department of Forest Protection, Unit of Phytopathology, Vienna, Austria


-
-
Taxonomy
Kingdom. Fungi · Phylum. Ascomycota · Class. Sordariomycetes · Order. Xylariales · Family. Xylariaceae · Genus. Cryptostroma P.H.Greg. & S.Waller. · Species. Cryptostroma corticale (Ellis & Everh.) P.H. Greg. & S. Waller, 1951
Synonyms
Coniosporium corticale Ellis & Everh., 1889
Local names
Sooty bark disease (SBD; English), Enfermedad de la corteza de hollín (Spanish), Maladie de la suie (French), Rußrindenkrankheit (German), Sazná nemoc kůry (Czech)
-
-
-
The anamorphic fungus Cryptostroma corticale was first isolated from sugar maple (Acer saccharum Marshall) logs in 1889 (Ellis & Everhart, 1889) in Canada. Afterwards, the following record of the fungus was already in Europe on sycamore (A. pseudoplatanus), hickories (Carya spp.) and basswoods (Tilia spp.) (Gregory, Peace and Waller, 1949; Gregory and Waller, 1951) as well as in North America on maple (Acer sp.), (Gregory and Waller, 1951). Further notifications of SBD were associated with other species of maple such as field maple (A. campestre L.) (Moreau and Moreau, 1954), Norway maple (A. platanoides L.) (Moreau and Moreau, 1954; Bencheva, 2014), and ash-leaved maple (A. negundo L.) (Moreau and Moreau, 1954; Young, 1978), red maple (A. rubrum) (Washington State Department, 2021) and more recently to bigleaf maple (A. macrophyllum) (Brooks et al., 2023) and other Acer spp. (Brooks et al., 2022). Other tree species have been also identified as potential tree hosts but might be affected to a lesser extent such as Aesculus hippocastanum L. (Young, 1978; Brooks et al., 2022; Brenken et al., 2024), Betula spp. (Cochard et al., 2015; Brooks et al., 2022) or Fraxinus excelsior (Langer et al., 2023).
-
-
-
This fungus is known only in its asexual phase (Dickenson 1980; Kelnarová et al., 2017) and its spread is through the asexual spores called conidia. These airborne conidia might mainly enter in the host tree through open wounds (Dickenson, 1980; Kelnarová et al., 2017) but seems also to have the ability to penetrate through intact bark (Bußkamp et al., 2024). Initially it infects the heartwood and subsequently the sapwood, cambium and phloem. The spread is mainly in the longitudinal direction of the trunk (Cech, 2018). Primarily it remains as an endophyte, saprophyte or latent invader and the tree remains asymptomatic (Kelnarová et al., 2017; Ogris et al., 2021; Brooks et al., 2023; Schlößer et al., 2023). When the host is weakening or the weather conditions are favorable for the fungus, it becomes an opportunistic pathogen which eventually kills the tree in a fast tempo. The aggressiveness and fast spread of this fungus prevails over other fungi as demonstrated in pathogenicity trials (Bußkamp et al., 2024). Ideal conditions for its pathogenic stage imply high summer temperatures and drought stress, being its optimal growth temperature of 25 °C (Dickenson, 1980). Therefore, increased number of SBD outbreaks are presumed to occur in accordance with the climate change predictions. As first signs of infection, branch dieback, wilting of leaves and epicormic shoots are typical and these early symptoms might have a slow progression (Gregory and Waller, 1951; Brooks et al., 2023; Tanney et al., 2024). Extensive damage might occur after warm summers (Abbey, 1978) as the fungus invades the cambium and the phloem of affected trees. In these advanced stages, the pathogen reaches the outer bark producing extensive necroses of subcortical tissues and developing subcortical stromata. The shedding of necrotic bark exposes the brown-black stromata (fruiting bodies) with conidiophores associated with masses of soot-like spores (Gibbs, 1997). These masses of conidia produced from the stromata under the bark gave rise to the name “sooty bark disease”. The peeled areas of dying branches or already dead trees remain covered with the black stroma even after dispersion of the wind-blown spores (Gregory and Waller, 1951; Koukol et al., 2015). After felling the tree, visual symptoms of the infection can be observed by the discoloration of the heartwood and sapwood (Koukol et al., 2015; Ogris et al., 2021). This discoloration is characterized by a greenish-brown, sharply edged stain (Gregory and Waller, 1951; Ogris et al., 2021) as a result of the secondary metabolism-defense of the tree against a broader range of fungal pathogens. However, this stain disappears after the tree dies (Gregory and Waller, 1951).


-
-
-
C. corticale was first isolated from maple, most probably sugar maple (A. saccharum Marshall) firewood in London, Ontario (Canada) in 1889 and called Coniosporium corticale Ellis & Everh. (Ellis & Everhart, 1889). It was described as a non-parasitic fungus. Other reports of the fungus were also restricted to the Great Lakes region, particularly in Wisconsin and Michigan during the first half of the 1900s (Towey et al., 1932; Gregory and Waller, 1951). Meanwhile, in Europe this fungus was isolated in Essex (UK) from sycamore (A. pseudoplatanus) by Gregory, Peace and Waller (1949) but remained unidentified until the study performed by Gregory and Waller (1951) where it was firstly described as C. corticale. This study was performed in Wanstead Park in London, where the fungus was observed on one dead sycamore tree in 1945 and in about 40 dead or dying sycamores in 1948. Subsequent surveys in 1949 and 1950 revealed the occurrence of infections in more than 150 trees. Apart from this particular outbreak in UK, along the 20th century only occasional epidemics were described in France (Moreau and Moreau, 1954), Italy (Wilkins, 1952) and Germany (Plate and Schneider, 1965). In France, Moreau and Moreau (1954) described the occurrence of SBD not only in sycamore but also in Norway maple, field maple and one case in an ash-leaved maple. During the last century the incidence of SBD was increasing and the spread of the pathogen in Europe is notorious with several records in Central Europe (Cech, 2004, 2019; Metzler, 2006), south-eastern Europe (Bencheva, 2014), southern Europe (Longa et al., 2016) and eastern Europe (Gninenko et al., 2024).
-
-
-
C. corticale is putatively native to the Great Lakes Region of North America (Brooks et al., 2022; Muller et al., 2023). It is widely spread in the North America, and in Europe where is now considered an invasive pathogen (EPPO; 2024). After its first observation in Ontario, Canada and in the USA (Towey et al., 1932; Gregory and Waller, 1951), further records appeared in Europe, specifically, in the UK (Gregory and Waller, 1951) followed by Italy (Wilkins, 1952) and France (Moreau and Moreau, 1954). In the last decades, the distribution of SBD is extended to other countries such as Czech Republic (Koukol et al., 2015; Kelnarová et al. 2017), Austria (Cech, 2004; 2019), Belgium (Vaïanopoulos and Schmitz, 2023), Bulgaria (Bencheva, 2014), Germany (Metzler, 2006; Robeck, 2007), Slovenia (Ogris et al., 2021), Switzerland (Cochard et al., 2015), The Netherlands (EPPO, 2014) and Russia (Gninenko et al., 2024). Within the USA, it has been recorded in the states of Wisconsin, Michigan (Towey et al., 1932; Gregory and Waller, 1951), Washington (Washigton State Department 2021; Brooks et al., 2023) and more recently in California (Garbelotto et al., 2024). Canada has also reported new spots of maples affected by SBD in British Columbia (Tanney et al., 2024). Due to its latent/endophytic stage in apparently asymptomatic trees, the current distribution of C. corticale might be wider (Cech, 2018). Furthermore, the increasing threshold of the mean monthly daily maximum temperatures during summers will favor the spread of the pathogen and new outbreaks in northern countries (Cech, 2018).
-
-
-
Long range
The frequent number of records over the last decades appears to show the great ability of the pathogen to spread (EPPO 2023). Its aerial spores (conidia) are able to be dispersed with wind over 300 km according to the aerobiological surveillance performed with active traps by Muller et al. (2023). The study of Muller et al. (2023) was the first to prove the efficiency of the conidia to be dispersed over long distances.
Short range
Despite the great mobility of this aerial pathogen, it appears to remain as an endophyte inside its host tree until occurring favorable conditions such as extreme heat and drought during summer. Local dispersion might be facilitated not only by the wind but also by animal vectors. Squirrels are known for their bark-stripping behaviour and by stripping the bark to expose stromata of symptomatic trees, they would carry the spores and transport them to other trees by creating entry wounds (Tanney et al., 2024). Thus, Tanney et al (2024) observed teeth marks in many stromata and strips of bark from symptomatic sycamore trees standing on the ground. Abbott et al., (1977) proved that the C. corticale conidia collected from buccal cavity, claws and hindgut was viable and able to germinate on malt extract agar. Dispersion by birds such as woodpeckers might also be a plausible mode (Kelnarová et al., 2017 Operational activities during the management of the affected trees are also contributing to the dispersion at a local range. Felling and pruning of the trees should be done in winter and early spring and at dump weather as the sporulation rate is the lowest (Bencheva, 2014; Burgdorf et al., 2022) and precipitation wash out the spores preventing the wind dispersion (Bork, 2018). The use of disposable clothing material for the operation prevents also the spread of the fungus.
-
-
-
C. corticale is currently considered a non-quarantine and non-regulated pest according to the European and Mediterranean Plant Protection Organization (EPPO). The maple bark disease (MBD) caused by C. corticale is recognized as an occupational disease in Germany, Austria and Switzerland (Braun et al., 2021).
-
-
-
Ecological Impacts
C. corticale has been for some years a big threat to maples (Acer spp.) through Europe with continuous new records of damages (Bußkamp et al., 2024). The most susceptible genus to SBD is Acer and within Europe A. pseudoplatanus and A. platanoides are notoriously associated with SBD outbreaks. In addition, many other fungal and oomycete pathogens are known to affect the vitality of maple such as Nectria (Hiemstra 1998; Račko et al., 2022), Verticilium dahliae (Sinclair et al., 1981; Brglez et al., 2024), Eutypella parasitica (Cech et al., 2016; Brglez et al., 2020) or Phytophthora spp. (Ginetti et al., 2014; Milenković et al., 2014). A. pseudoplatanus is native to Central Europe and typical in mixed forests with high requirements of nutrients and water supply while A. platanoides is more modest in terms of needs.
Economic Impacts
C. corticale in an endophytic stage should not affect the wood quality of as shown in the studied areas by Schlößer et al. (2023). In contrast, when the fungal becomes pathogenic and tongue-shaped necroses are developed on the surface of the trunk, typical extensive greenish-grey wood discoloration and wood rot are observed in the inner trunk after felling the trees. That implies a complete devaluation of the trunk wood (Grüner et al., 2020; Kesphol et al., 2022). For the moment, SBD has been prevalently reported from municipal areas (e.g. Gregory and Waller, 1951; Bencheva, 2014; Koukol et al., 2015). It is probably due to higher air temperature in cities and higher incidence of wounds acting as infection gates. Therefore, the high occurrence of SBD in public green enhances the cost for its maintenance and management.
Social Impacts
The conidia of C. corticale, which are produced in vast masses up to 170.000.000 spores/cm2 (Gregory and Waller 1951; Bork, 2018), have a particular impact on human health. These spores are the causal agent of the maple bark disease (MBD) (Emanuel et al., 1962), or also defined by WHO as Maple Bark Stripper Lung (WHO, 2022). It is hypersensitivity pneumonitis caused by inhalation of the spores with symptoms such as allergic asthma, flu-like infections and interstitial pneumonia (Braun et al., 2021). This disease is a hazard for those occupations such as arborists, gardeners, woodman, foresters, sawyers or paper mill workers who are exposed to the spore inhalation for long periods due to activities such as removing and handling infected wood. Occasional recreational activities in areas with SBD and limited exposure to the spores are considered a low health risk for most people (Braun et al. 2021). The first cases were already mentioned in 1932 and it was defined as a diffuse lung disease affecting lumber workers peeling the trunk of diseased maples in the USA (Towey et al., 1932). Later, in 1962, it was described in detail as the maple bark disease (MBD) (Emanuel et al., 1962). Upon a proper diagnosis and therapy in time, the recovery from MBD is complete, but chronic disease could lead to lung fibrosis and death (Braun et al., 2021). It is therefore recommended to wear personal protective equipment (goggles, particle filtering respiratory systems FP3, Protective suit with hood, protective gloves and boots) to avoid direct contact with spores of C. corticale for occupation (Niesar et al., 2020; Braun et al., 2021; Burgdorf et al., 2023). Furthermore, felling of the affected trees should be done with a harvester and not with a chainsaw to avoid closer contact with the spores (Burgdorf et al., 2023).


-
-
-
Detection
The ability of this pathogen to remain in a latent stage as an endophytic fungus or mild pathogen makes it to be unnoticed in asymptomatic trees. The future threats of susceptible hosts under these climate change scenarios urge the application of early detection methods for identifying these latent infections of C. corticale. DNA-based approaches are then essential for early detection (Kelnarová et al., 2017; Brooks et al., 2023). Sampling of asymptomatic living trees for applying molecular detection or cultivation methods consists of extraction of wooden cores with an increment borer (Kelnarová et al., 2017; Schlößer et al., 2023; Brooks et al., 2023). Traditional culture cultivation of the wooden cores serves as a method to isolate the pathogen; however, DNA-methods are more sensitive for confirming the presence of C. corticale in wood tissue.
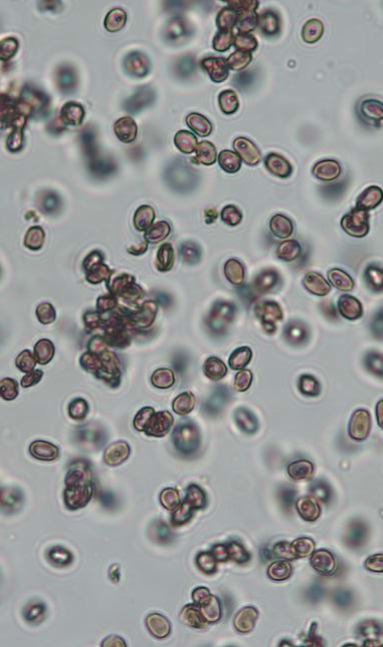
Although identification of SBD stroma of typical brown-black colour by naked eye is possible, it is sometimes difficult due to very common presence of other mostly saprobic fungi creating black stroma (Hülsewig, 2019). Namely, Eutypa maura or Stegonsporium pyriforme produce stroma on the surface of dying or dead stems of sycamores. It is always recommended to confirm the determination of the stroma by microscopy and identify typical brown, ovoid (or sometimes distorted due to pressure) conidia, 4-6.5 x 3.5-4 µm in size (Gregory and Walles, 1951; Ogris et al., 2021).
Spore trapping is an effective method for early detection and surveillance of airborne fungal pathogens. Burgdorf et al. (2022) studied the influence of season and vertical factors on abundance of conidia by installation of passive spore traps at different heights and distances from the host tree. Muller et al (2023) performed an extensive aerobiological study using 12 active vacuum-pumped suction traps (Hirst-type air samplers) from the existing European pollen-monitoring network in 6 countries. They also developed a species-specific quantitative PCR assay to detect and quantify the spores from these C. corticale aerobiological samples.
Preventive measures and suppression measures
Heat and drought are the main triggers of the SBD development and expression (Cech 2004; Metzler 2006; Bencheva, 2014). Furthermore, other stressful factors such as floodings and those associated with urban environments, like pruning and accidental wounding, together with soil compression promote SBD (Koukol et al., 2015; Kelnarová et al., 2017). Therefore, susceptible hosts such as sycamore trees located in urban areas should be supplied with their demands of nutrients and water for the maintenance of vitality of the tree (Bork, 2018). Especially during drought and high temperature periods, when the disease risk is high, it is important to provide enough irrigation to susceptible hosts (Tanney et al., 2024). Likewise, yearly surveillance for observation of infection symptoms is suggested, notably during these heat and drought periods, when outbreaks occur. Especial attention should be paid to upper branches which can be infected but unnoticed during visual assessments (Kelnarová et al., 2017).
The choice of other less susceptible tree species to SBD or less demanding in terms of nutrients and water should be also considered for the planning of suitable species for planting and reforestation programs, especially in hands with the future climate change predictions. Within urban settings, non-host tree species should be prioritized for planting (Tanney et al 2024). When planting susceptible maple species, selection for those ones more endured is to prevail. Thus, although the Norway maple is also a susceptible maple species, it requires less supply of nutrients and water than sycamore and therefore, its planting should be more suitable for certain sites (Grüner et al., 2020).
In general, removal of SBD-affected trees from the area seems to slow down the spread of the pathogen (Kelnarova et al., 2017) although at an endophytic state with asymptomatic infected trees (Schlößer et al., 2023), they might remain unnoticed. Furthermore, felled infected trees are reported to be source of new infections (Gregory and Waller, 1951). This implies a source for sporulation and spread of the pathogen to other trees and areas. Therefore, the stumps from infected trees should be removed and either buried on-site or transported in covered containers for subsequent incineration. It is also recommended to schedule pruning and felling in winter and early spring (Burgdorf et al., 2022) and under damp weather conditions (Bork, 2018), when the sporulation is minimal, and the conditions are not conducive for the spore dispersion (Burgdorf et al., 2022). It is important to note that infested wood is unsuitable for firewood and should not be chipped to prevent the spread of spores (Bork, 2018; Niesar et al., 2019; Tanney et al., 2024). Also, if the wood does not show any sign of infection but is still collected from a SBD site, it should be discarded for firewood because it can develop a stromata during its storage (Plate and Schneider, 1965; Burgdorf et al., 2023).
-
-
-
1. Abbott, R.J., Bevercombe, G.P., Rayner, A.D.M. (1977). Sooty bark disease of sycamore and the grey squirrel. Trans Br Mycol Soc. 69(3):507–508.
2. Bencheva, S. (2014). First report of Cryptostroma corticale (Ellis & Everh.) P.H. Greg. & S. Waller on Acer platanoides L. in Bulgaria. Silva Balcania 15(2):101–104.
3. Bork, K. (2018). Rußrindenkrankheit an Ahorn – Erstfund in Bayern. AZV Der Wald 20/2018. (https://www.waldwissen.net/assets/waldwirtschaft/schaden/pilze_nematoden/lwf_russrinde/download/AFZ_20_18_Russrindenkrankheit.pdf; accessed on 24 September 2024).
4. Brglez, A., Devetak, Z., Ogris, N., Radišek, S., & Piškur, B. (2024). An outbreak of Verticillium dahliae on sycamore maple in a forest stand in Slovenia. Journal of Plant Pathology, 1-13.
5. Brglez, A., Piškur, B., & Ogris, N. (2020). Eutypella parasitica and other frequently isolated fungi in wood of dead branches of young sycamore maple (Acer pseudoplatanus) in Slovenia. Forests, 11(4), 467.
6. Brenken, A. C., Kehr, R., Riebesehl, J., Esch, J., & Enderle, R. (2024). First report of Cryptostroma corticale on Aesculus hippocastanum causing sooty bark disease in Germany. Journal of Plant Diseases and Protection, 131(3), 1087-1092.
7. Brooks, R., Hulbert, J. M., Omdal, D., Elliott, M., & Chastagner, G. A. (2022). Sooty bark disease diagnostic guide. Washington State University Extension Publications.
8. Brooks, R. K., Omdal, D., Brown, S., Marshall, C. J., Hulbert, J. M., Elliott, M., & Chastagner, G. (2023). Cryptostroma corticale, the causal agent of sooty bark disease of maple, appears widespread in western Washington State, USA. Forest Pathology, 53(6), e12835.
9. Burgdorf, N., Härtl, L., & Hahn, W. A. (2022). Sooty Bark Disease in Sycamore: Seasonal and Vertical Variation in Spore Release of Cryptostroma corticale. Forests, 13(11), 1956.
10. Burgdorf, N., Straßer, L., & Hahn, W. A. (2023). Ahorn-Rußrindenkrankheit - LWF Merkblatt 52
11. Bußkamp, J., Bien, S., Neumann, L., Blumenstein, K., Terhonen, E., & Langer, G. J. (2024). Endophytic community in juvenile Acer pseudoplatanus and pathogenicity of Cryptostroma corticale and other associated fungi under controlled conditions. Journal of Plant Pathology, 1-13.
12. Cech, T. L. (2004). Bemerkenswerte Krankheiten in 2004. Forstschutz Aktuell, 32, 31–34.
13. Cech, T.L. (2018). Rußrindenkrankheit bedroht Ahornbestände in Laubwäldern im Osten Niederösterreichs. Forstschutz Aktuell 65(4):1–6 (https://bfw.ac.at/rz/bfwcms.web?dok=10373)
14. Cech, T. L. (2019). Rußrindenkrankheit bedroht Ahornbestände in Laubwäldern im Osten Niederösterreichs. Forstsch Aktuell, 65, 24.
15. Cech, T. L., Schwanda, K., Klosterhuber, M., Straßer, L., & Kirisits, T. (2016). Eutypella canker of maple: first report from Germany and situatio
16. Cochard, B., Crovadore, J., Bovigny, P.Y., Chablais, R., Lefort, F. (2015). First reports of Cryptostroma corticale causing sooty bark disease in Acer sp. in Canton Geneva, Switzerland. New Dis Rep 31:8.
17. Emanuel, D.A., Lawton, B.R., Wenzel, F.J. (1962). Maple-bark disease. Pneumonitis due to Coniosporium corticale. The New England Journal of Medicine 26(7): 333–337.
18. EPPO. (2014). First report of Cryptostroma corticale in the Netherlands. European Plant Protection Organisation Reporting Service 2014/7: 133. (https://gd.eppo.int/reporting/Rse-2014-07).
19. EPPO. (2023). Sooty bark disease of sycamore is spreading in Europe. European Plant Protection Organisation Reporting Service 2023/9: 211. (https://gd.eppo.int/reporting/article-7693).
20. EPPO Global Database. (2024). Cryptostroma corticale. (https://gd.eppo.int/taxon/CRPSCO/distribution; last updated: 2024-07-08).
21. Garbelotto, M., Popenuck, T., Schmidt, D., Rooney-Latham, S., Ewing, C., & Smith, T. (2024). First report of Cryptostroma corticale causing sooty bark disease in California and first worldwide report of silver maple as a host. Plant Disease, 108(5), 1395.
22. Ginetti, B., Moricca, S., Squires, J. N., Cooke, D. E. L., Ragazzi, A., & Jung, T. (2014). Phytophthora acerina sp. nov., a new species causing bleeding cankers and dieback of Acer pseudoplatanus trees in planted forests in northern Italy. Plant Pathology, 63(4), 858-876.
23. Gninenko, Y. I., Chilakhsaeva, E. A., Seraya, L. G., Larina, G. E., Yufereva, V. V., Bondareva, E. V., & Yarylchenko, T. N. (2024). First Report of Cryptostroma corticale, a causative agent of the Sooty Bark Disease of Maples, in Russia. Russian Journal of Biological Invasions, 15(1), 26-31.
24. Gregory, P. H., Peace, T. R. & Waller, S. (1949). Death of sycamore trees associated with an unidentified fungus. Nature, Lond., I64, 275.
25. Grüner, J., Berens, A., Delb, H. (2020). Die Ahorn-Rußrindenkrankheit in Südwestdeutschland: Gefahren, Prognose und Empfehlungen. Waldschutz-info 2/2020, 8 S. (https://www.waldwissen.net/de/waldwirtschaft/schadensmanagement/pilze-und-nematoden/ahorn-russrindenkrankheit; accessed on 24 September 2024).
26. Herausforderung. IPA-Journal 01/2022, S. 26-30. (https://www.dguv.de/medien/ipa/publikationen/ipa-journale/ipa-journale2022/ipa_journal_1_2022_cryptostroma.pdf; accessed on 03 December 2024)
27. Hülsewig, T. (2019). Cryptostroma corticale und Verwechslungsarten. (https://www.pilzforum.eu/attachment/294988-cryptostroma-corticale-und-verwechslungsarten-t-hülsewig-2019-pdf/; accessed on 03 December 2024)
28. Kelnarová, I., Černý, K., Zahradník, D., & Koukol, O. (2017). Widespread latent infection of Cryptostroma corticale in asymptomatic Acer pseudoplatanus as a risk for urban plantations. Forest Pathology, 47(4), e12344.
29. Kespohl Sabine, Grüner Jörg, Enderle Rasmus, Riebesehl Janett, Raulf Monika (2022): Exogen allergische Alveolitis (EAA) durch den Erreger der Rußrindenkrankheit (Cryptostroma corticale) – Eine diagnostische
30. Koukol, O., Kelnarová, I., & Černý, K. (2015). Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. Forest Pathology, 45(1), 21-27.
31. Langer, G. J., Peters, S., Bußkamp, J., & Bien, S. (2023). Cryptostroma corticale and fungal endophytes associated with Fraxinus excelsior affected by ash dieback. Journal of Plant Diseases and Protection, 1-11.
32. Longa, C. M. O., Vai, N., Maresi, G. (2016). Cryptostroma corticale in the northern Apennines (Italy). Phytopathologia Mediterranea, 55: 136–138.
33. Metzler, B. (2006). Cryptostroma corticale on Acer pseudoplatanus in Germany after the drought period of 2003. Mitt. Biol. Bundesanst. Land Forstw 400, 161–162.
34. Milenković, I., Nowakowska, J. A., Oszako, T., Mladenović, K., Lučić, A., Rakonjac, L., & Karadžić, D. (2014). Morphological and molecular identification of Phytophthora species from maple trees in Serbia. Genetika, 46(2), 353-368.
35. Moreau, C., Moreau, M. (1954). Nouvelles observationes sur le deperissement des erables. Bull Société Linn Normandie 7:6–67.
36. Muller, E., Dvořák, M., Marçais, B., Caeiro, E., Clot, B., Desprez-Loustau, M. L., ... & Gomez-Gallego, M. (2023). Conditions of emergence of the Sooty Bark Disease and aerobiology of Cryptostroma corticale in Europe. NeoBiota, 84, 319-347.
37. Ogris, N., Brglez, A., & Piškur, B. (2021). Drought stress can induce the pathogenicity of Cryptostroma corticale, the causal agent of sooty bark disease of sycamore maple. Forests, 12(3), 377.
38. Plate, H. P., Schneider, R. (1965). Ein Fall von asthmaartiger Allergie, verursacht durch den Pilz Cryptostroma corticale. Nachrichtenblatt des deutschen Pflanzenschutzdienstes, 17(7), 100-101.
39. Robeck, P. (2007). Die Russrindenkrankheit (Cryptostroma corticale) des Ahorns in Deutschland. Leipzig: Grin Verlag.
40. Schlößer, R., Bien, S., Langer, G. J., & Langer, E. J. (2023). Fungi associated with woody tissues of Acer pseudoplatanus in forest stands with different health status concerning sooty bark disease (Cryptostroma corticale). Mycological Progress, 22(2), 13.
41. Sinclair, W. A., Smith, K. L., & Larsen, A. O. (1981). Verticillium wilt of maples: symptoms related to movement of the pathogen in stems. Phytopathology, 71(3), 340-345.
42. Tanney, J. B., Feau, N., Shamoun, S. F., Kope, H. H., Dicaire, A., Drugmand, B., ... & Joshi, V. (2024). Cryptostroma corticale (Ellis & Everh.) PH Greg. & S. Waller causing sooty bark disease in British Columbia, Canada. Canadian Journal of Plant Pathology, 1-15.
43. Towey, J.W., Sweany, H.C., Huron, W.H. (1932). Severe bronchial asthma apparently due to fungus spores found in maple bark. J Amer Med Assoc., 99:453–459.
44. Vaïanopoulos, C., Schmitz, S. (2023). Maladie de la suie de l'érable. (https://owsf.environnement.wallonie.be/fr/maladie-de-la-suie-de-l-erable.html?IDD=6755&IDC=5642)
45. Washington State Department of Natural Resources (DNR), Forest Health and Resiliency Division. (2021). Forest health highlights in Washington/2020. A summary of insect, disease, and other disturbance conditions affecting Washington’s forests.
46. World Health Organisation. (2022). CA70.6 Maple bark stripper lung. ICD-11 for Mortality and Morbidity Statistics.
47. Wilkins, V.E. (1952). Report of the technical Working Party, EPPO, Paris, 19 pp.
48. Young, C.W.T. (1978). Sooty Bark Disease of Sycamore (Arboricultural Leaflet). Stationery Office Books, Department of Environment, London u.a
-
-